Perinatal hypoxia is a leading cause of morbimortality worldwide. Up to 40% of newborns who experience oxygen deprivation suffer long term neurological impairment. The impact of a hypoxic brain injury has been well investigated; however, despite its functional importance and immaturity at birth, the involvement of the cerebellum remains poorly understood.
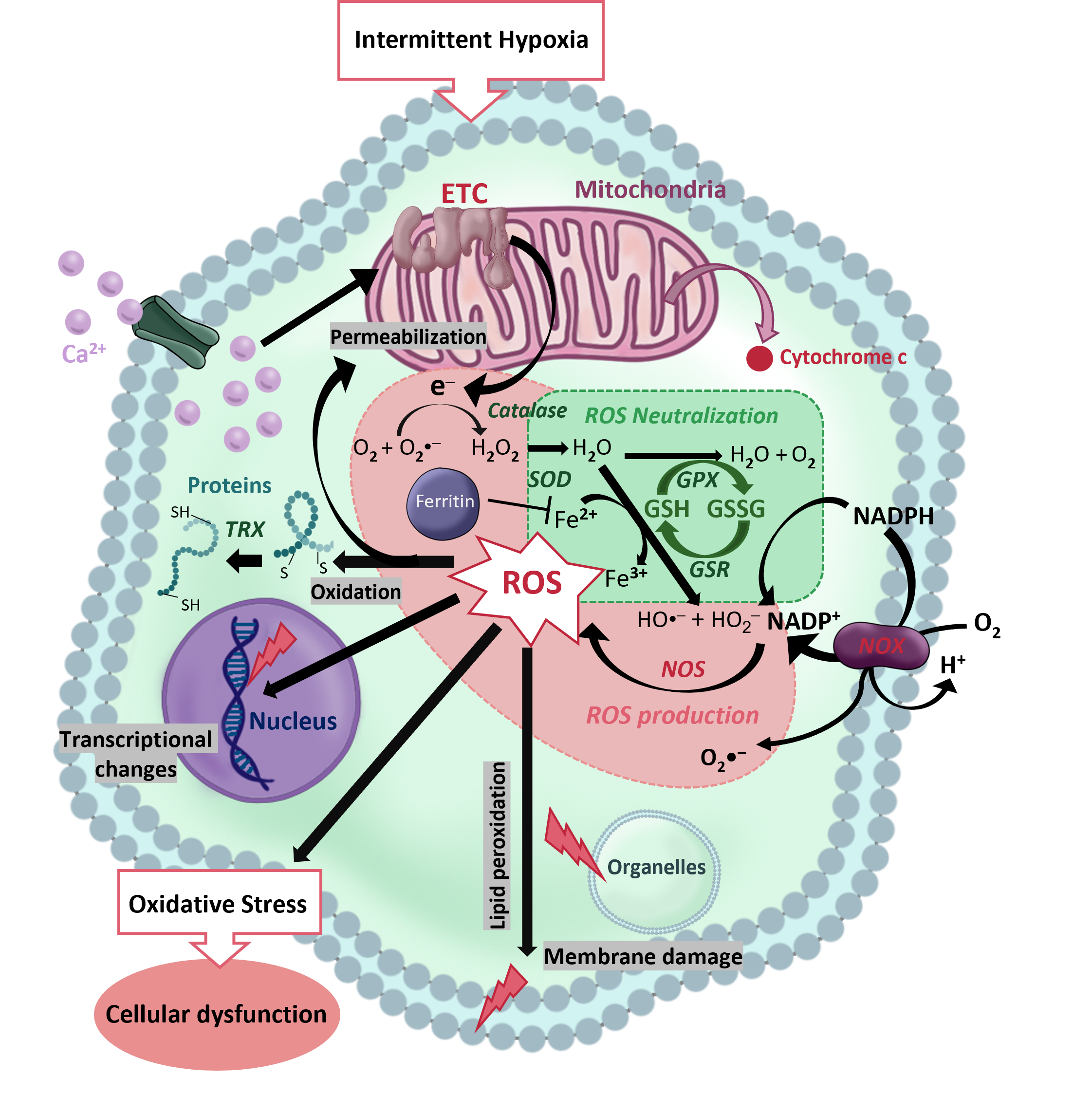
A major cellular consequence of hypoxia is Oxidative Stress (OS), a metabolic state caused by the accumulation of Reactive Oxygen Species (ROS), mainly upon reoxygenation. The generation, neutralization and effects of ROS encompass a complex web of intracellular pathways. This work aims at shedding light on the genes involved in the response to OS in the cerebellum. To this end, we studied the cerebellar expression of a panel of genes related to OS by RT-PCR on two murine models. i) A continuous hypoxia (5% O2) during a single 40-minute episode at postnatal day 12 (P12) designed to mimic perfusion pathologies or respiratory delay at birth, and ii) an intermittent hypoxia (IH), consisting of repeated 2-minute cycles of hypoxia-reoxygenation (20 seconds at 5% O2) between P2 and P12 during 6 hours per day, and constituting a valid model of Apnea of Prematurity (AoP).
Our results indicate that IH induces stronger modifications of OS-related gene expression than continuous hypoxia, highlighting that the severity of the alteration is linked to the number of reoxygenation events. Indeed, we observed that, after IH, genes involved in ROS production are overexpressed while genes encoding antioxidant enzymes are underexpressed. These alterations could induce a failure of the defense system against ROS and, in turn, be responsible for neuronal death in the cerebellum. Interestingly, we also found that the expression of genes coding for several cytoskeleton-associated proteins is altered after IH. This suggests that IH could modify the phenotype of various neurons which could contribute to histological defects.
Taken together, our data indicate that, in terms of OS, AoP seems to be more deleterious than a single hypoxic episode. The alterations in the OS defense system and the neuronal phenotype could contribute to the neurodevelopmental disorders observed in human newborns. This work participates in understanding the underlying mechanisms of hypoxic cerebellar injury. In the long term, it could lead to the identification of novel therapeutic targets to address this socially and economically relevant health issue.