Apnea of Prematurity (AOP) is a cause of perinatal hypoxia and up to 50% of premature newborns are affected. The link between persistent AOP and long-term neurological impairment has been established and the impact of hypoxic brain injuries has been investigated. However, despite its functional importance and immaturity at birth, the involvement of the cerebellum remains poorly understood.
This work aims at shedding light on the mechanisms underlying cerebellar hypoxic injury. To this end, we developed a mouse model of AOP, consisting of repeated 2-minute cycles of hypoxia-reoxygenation, including 20 seconds at 5% O2, between P2 and P12, for 6 hours/day. Histological studies following this intermittent hypoxia (IH) protocol revealed a delay in cerebellar maturation and a modification of Purkinje cell development. These deficits are associated with long-term alterations in functions linked to the cerebellum such as learning and motor skills.
To further explore the molecular mechanisms involved in these defects, we performed a transcriptomic study of genes involved in oxidative stress and various steps of cerebellar development including proliferation, migration, and differentiation. We analyzed their expression in different developmental stages (P4, P8, P12 and P21) and in different cell types by using laser capture microdissection. We showed that all stages are affected by IH, but more markedly P8. Moreover, we revealed that HIF1α, known to control vascularization, is decreased in IH, suggesting that cerebellar angiogenesis could be affected. Thus, we demonstrated by RT-qPCR that the expression of several genes involved in angiogenesis and vascularization is downregulated by our IH protocol.
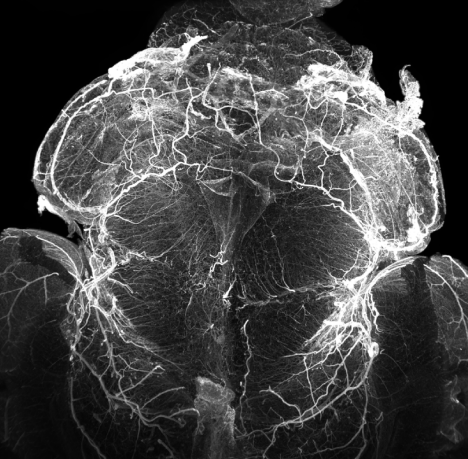
Moreover, we conducted immunohistochemical studies to observe the repercussions of these changes within the tissue. Preliminary findings show that the length and volume of vascular segments are decreased after IH. We are now conducting transparisation studies to observe the vasculature in 3D and expect to observe changes in the density and structure of blood vessels in response to IH. These findings could participate in explaining the pathophysiology of AOP.
Our present work provides elements to better understand the vascular aspects of AOP-induced cerebellar injury and could lead to novel targets to address this socio-economically relevant health issue.